Multiple Choice
Which period of elements,indicated by letter on the periodic table,has electrons whose highest principal quantum number n is 5? 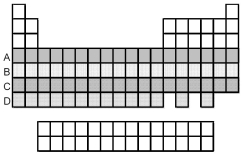
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q29: Which of the following elements will have
Q30: Which has the highest Z<sub>eff</sub> for its
Q31: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q32: How many electrons can a single orbital
Q33: An orbital with n = 5 and
Q35: Which of the following have their valence
Q36: Molybdenum has an anomalous electron configuration.Write the
Q37: Of the following,which atom has the largest
Q38: Using shorthand notation,the electron configuration of Ni
Q39: According to the Balmer-Rydberg equation,transitions from n