Multiple Choice
Which groups of elements,indicated by letter on the periodic table,have two unpaired p electrons in their valence shell? 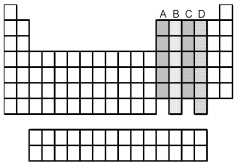
A) A and B
B) A and C
C) B and C
D) B and D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: The wave characteristics of a large,moving object,such
Q12: The spheres below represent atoms of Li,Be,B,and
Q13: An old copper penny has a mass
Q14: The property of a wave that is
Q15: For a multielectron atom,a 3s orbital lies
Q17: A quantized variable<br>A)can be continuously varied.<br>B)can only
Q18: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which grouping of
Q19: Which of the following represent electron configurations
Q20: The subshell designations follow the alphabet after
Q21: What is the de Broglie wavelength of