Multiple Choice
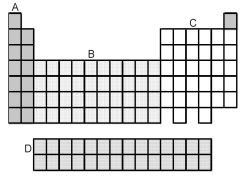
-Which grouping of elements,indicated by letter on the periodic table above,represents the f-block elements?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Atoms of which element,indicated by letter on
Q2: An oxygen molecule has a mass of
Q3: The number of orbitals in the n
Q5: The first vibrational level for NaH lies
Q7: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which element,indicated by
Q8: What are the possible values of n
Q9: Which group of elements,indicated by letter on
Q10: For an electron in a given atom,the
Q11: The wave characteristics of a large,moving object,such
Q47: How many subshells are there in the