Multiple Choice
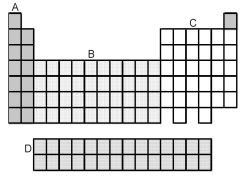
-Which grouping of elements,indicated by letter on the periodic table above,represents the p-block elements?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: For hydrogen,what is the wavelength of the
Q61: The number of orbitals having the quantum
Q62: According to the Bohr model,when a hydrogen
Q63: The four lines observed in the visible
Q64: The energy of an electron in a
Q67: The number of orbitals in a given
Q68: The intensity of a beam of light
Q69: How many subshells are there in the
Q70: Which orbitals have two nodal planes passing
Q71: The spheres below represent atoms of Sb,As,P,and