Multiple Choice
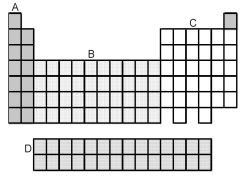
-Which grouping of elements,indicated by letter on the periodic table above,represents the s-block elements?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q94: The average bond dissociation energy of a
Q95: What is the ground-state valence-shell electron configuration
Q96: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q97: Which of the following does not have
Q98: Which of the following represent electron configurations
Q100: In a representation of an orbital,a region
Q101: An orbital that as the appearance of
Q102: Which of the following is true? The
Q103: How many valence electrons does a neutral
Q104: A solution to the Schrödinger wave equation