Multiple Choice
The spheres below represent atoms of Sb,As,P,and N (not necessarily in that order) . 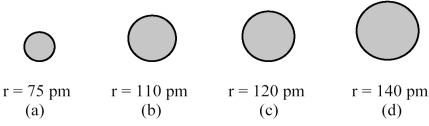
-Which one of these spheres represents an atom of N?
A) sphere (a)
B) sphere (b)
C) sphere (c)
D) sphere (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q128: Molecular vibrational energy transitions are observed in
Q129: What is the ground-state valence-shell electron configuration
Q130: Light behaves as if it were a
Q131: Which orbital-filling diagram represents the ground state
Q132: The spheres below represent atoms of Sb,As,P,and
Q134: Which atom in each group (I and
Q135: According to the Balmer-Rydberg equation,electromagnetic radiation with
Q136: How many h orbitals are allowed in
Q137: Which orbital-filling diagram violates the Pauli exclusion
Q138: Which has the highest Z<sub>eff</sub> for its