Multiple Choice
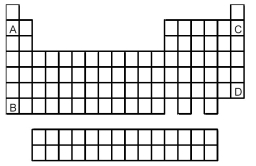
-Atoms of which element,indicated by letter on the periodic table,are expected to have the largest atomic radius?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q67: The number of orbitals in a given
Q68: The intensity of a beam of light
Q69: How many subshells are there in the
Q70: Which orbitals have two nodal planes passing
Q71: The spheres below represent atoms of Sb,As,P,and
Q73: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which of the
Q74: What is the ground-state electron configuration of
Q75: Copper has the anomalous electron configuration _.
Q76: What is the number of spherical nodes
Q77: List all the elements that have a