Multiple Choice
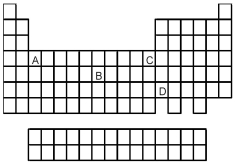
-Which element,indicated by letter on the periodic table above,has a 1+ ion with the electron configuration 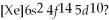
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q107: Which of the following represents the change
Q108: The ionic radius of Cs<sup>+</sup> is _
Q109: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q110: Which of the following ionic compounds would
Q111: What is the general trend in ionization
Q113: Which of the following species will have
Q114: Which of the following ionic compounds would
Q115: Which contains both covalent bonds and ionic
Q116: Of the following,which element has the highest
Q117: The octet rule is most likely to