Multiple Choice
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 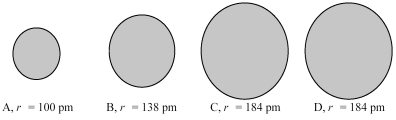
-Which sphere most likely represents the Ca2+ ion?
A) A
B) B
C) A or B
D) C or D
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Calculate the energy change for the formation
Q2: The four spheres below represent K<sup>+</sup>,Ca<sup>2+</sup>,Cl<sup>-</sup>,and S<sup>2-</sup>,not
Q4: How many electrons are in the outermost
Q5: Give the electronic configuration for Mn<sup>2+</sup>.<br>A)[Ar]2s<sup>2</sup>2p<sup>6</sup>3d<sup>5</sup><br>B)[Ar]4s<sup>2</sup>3d<sup>3</sup><br>C)[Ar]4s<sup>1</sup>3d<sup>4</sup><br>D)[Ar]3d<sup>5</sup>
Q6: Which ion does not have a noble
Q7: Which contains ionic bonds?<br>A)CH<sub>4</sub><br>B)CaCl<sub>2</sub><br>C)Cl<sub>2</sub><br>D)NCl<sub>3</sub>
Q8: Which element has the highest first electron
Q9: The following four spheres represent a metal
Q10: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q11: Consider Li<sup>+</sup>,F<sup>-</sup>,and O<sup>2-</sup>.Which ratio should be the