Multiple Choice
The four spheres below represent K+,Ca2+,Cl-,and S2-,not necessarily in that order. 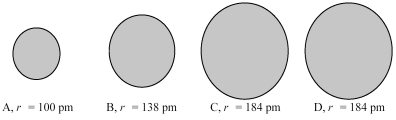
-Which sphere most likely represents the Cl⁻ ion?
A) A
B) B
C) A or B
D) C or D
Correct Answer:

Verified
Correct Answer:
Verified
Q71: Isoelectronic means having the same number of
Q72: Arrange the ions N<sup>3-</sup>,O<sup>2-</sup>,Mg<sup>2+</sup>,Na<sup>+</sup>,and F<sup>-</sup> in order
Q73: The element in period 3 with the
Q74: Which element has the least favorable (least
Q75: The following pictures represent alkali halide salts.
Q77: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Atoms of which
Q78: The following four spheres represent a metal
Q79: Using shorthand notation,the ground-state electron configuration of
Q80: Calculate the electron affinity for the formation
Q81: Which ion does not have a noble