Multiple Choice
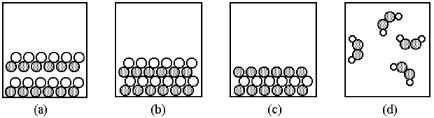
-Which of the above pictures are more likely to represent covalent compounds?
A) pictures (a) and (b)
B) pictures (a) and (d)
C) pictures (b) and (c)
D) pictures (b) and (d)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q80: Calculate the electron affinity for the formation
Q81: Which ion does not have a noble
Q82: Calculate the lattice energy for NaCl(s)using a
Q83: Using shorthand notation,the ground-state electron configuration for
Q84: Which of the following atoms with the
Q86: Indicate which is larger in each of
Q87: An element M reacts with chlorine to
Q88: Which contains covalent bonds?<br>A)LiOO<br>B)NH<sub>3</sub><br>C)LiCl<br>D)CaI<sub>2</sub>
Q89: Which of the following species will have
Q90: What is the ground-state electron configuration of