Multiple Choice
Based on the indicated electronegativities,arrange the following in order of increasing ionic character: CsBr,LaBr3,PBr3,MgBr2. 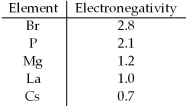
A) CsBr,LaBr3,MgBr2,PBr3
B) CsBr,MgBr2,PBr3,LaBr3
C) PBr3,LaBr3,MgBr2,CsBr
D) PBr3,MgBr2,LaBr3,CsBr
Correct Answer:

Verified
Correct Answer:
Verified
Q23: Which of the following contains an atom
Q24: At the equilibrium bond length<br>A)the attractive forces
Q25: In general,at room temperature<br>A)ionic compounds are all
Q26: Elements that can accommodate more than eight
Q27: Which molecule contains the most easily broken
Q29: Using only the elements Mg,Cl,and/or P,give the
Q30: NO<sub>2</sub><sup>-</sup> is be expected to have<br>A)two single
Q31: Of H<sub>2</sub>CO and CO and CO<sub>2</sub>,the compound
Q32: In the best Lewis structure for CN<sub>
Q33: The number of Lewis electron dot resonance