Multiple Choice
Which is the most acceptable electron dot structure for N2H2?
A) H - 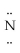
-
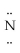
- H
B) H - 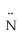
=
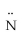
- H
C) H - N 
N - H
D) H - 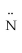

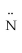
- H
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: How many lone pairs of electrons are
Q61: The compound CCl<sub>4</sub> contains<br>A)ionic bonds.<br>B)nonpolar covalent bonds.<br>C)polar
Q62: Of the following elements,which has the lowest
Q63: Which compound is most likely to exist
Q64: Which molecule contains the most polar bonds?<br>A)CF<sub>4</sub><br>B)TeO<sub>2</sub><br>C)NO<sup>-</sup><br>D)CCl<sub>4</sub>
Q66: How many lone pairs are on the
Q67: The phosphorus atom in PCl<sub>5</sub> would be
Q68: How many lone pairs are there in
Q69: Of the following elements,which has the highest
Q70: How many lone pairs are on the