Multiple Choice
Assign formal charges to each atom in the resonance form for SOCl2 given below. 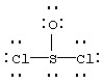
A) 0 for Cl,0 for S,and 0 for O
B) 0 for Cl,+1 for S,and -1 for O
C) -1 for Cl,+4 for S,and -2 for O
D) -1 for Cl,-2 for S,and -2 for O
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Based on formal charge considerations,the electron-dot structure
Q52: When melting S<sub>8</sub>,_ forces must be overcome
Q53: The Lewis electron-dot structure of H<sub>2</sub>CO has
Q54: Which of the following molecules is expected
Q55: The nitrogen-nitrogen bond in :N <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg"
Q57: Of the bonds C-C,C-N,C-O,and C-F,the bond that
Q58: How many lone pairs of electrons are
Q59: How many lone pairs in the correct
Q60: How many lone pairs of electrons are
Q61: The compound CCl<sub>4</sub> contains<br>A)ionic bonds.<br>B)nonpolar covalent bonds.<br>C)polar