Multiple Choice
Assign formal charges to each atom in the resonance form for SOCl2 given below. 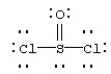
A) 0 for Cl,0 for S,and 0 for O
B) 0 for Cl,+1 for S,and -1 for O
C) -1 for Cl,+4 for S,and -2 for O
D) -1 for Cl,-2 for S,and -2 for O
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Which is expected to have the strongest
Q12: Which molecule contains a triple bond?<br>A)O<sub>2</sub><br>B)O<sub>3</sub><br>C)HCCH<br>D)Br<sub>2</sub>SO
Q13: How many double and single bonds are
Q14: Of the following elements,which has the lowest
Q15: The Lewis electron-dot structure of N<sub>2</sub> has
Q17: Which electron dot structure for OCN<sup>-</sup> has
Q18: What is the bond order of the
Q20: Covalent bonding is a<br>A)gain of electrons.<br>B)loss of
Q21: How many of the σ bonds in
Q67: The phosphorus atom in PCl<sub>5</sub> would be