Multiple Choice
Based on VSEPR theory,which should have the smallest XAX bond angle?
A) 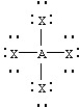
B) 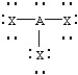
C) 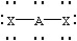
D) 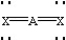
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q107: What is the geometry around the central
Q108: What is the molecular geometry of XeF<sub>4</sub>?<br>A)seesaw<br>B)square
Q109: In the molecule BF<sub>3</sub> there is a
Q110: Which is expected to have the largest
Q111: Which drawing represents a π bonding molecular
Q113: A triple bond is generally composed of<br>A)three
Q114: A single sp<sup>3</sup> hybrid orbital has<br>A)one lobe.<br>B)two
Q115: The bonds in the polyatomic ion NO<sub>3</sub><sup>-</sup>
Q116: A molecular model of BBr<sub>3</sub> is shown
Q117: What is the geometry around the central