Multiple Choice
What are the bond angles in the following molecular model of BrF5? 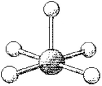
A) some less than 90° and some less than 120° but greater than 90°
B) 90° and 120°
C) some less than 90° and some less than 180° but greater than 120°
D) 90° and 180°
Correct Answer:

Verified
Correct Answer:
Verified
Q141: What is the molecular geometry of TeBr<sub>4</sub>?<br>A)seesaw<br>B)square
Q142: Which of the following best describes ICl<sub>2</sub><sup>-</sup>?
Q143: Which is expected to have the largest
Q144: Identify the set of hybrid orbitals shown
Q145: Which drawing best represents hydrogen bonding in
Q147: The carbon-carbon bond in C<sub>2</sub>H<sub>2</sub> contains _
Q148: The hybrid orbital used by nitrogen to
Q149: What is the geometry around the central
Q150: The number of sp<sup>2</sup> hybrid orbitals on
Q151: Shown below is a model of SiH<sub>4</sub>