Multiple Choice
A molecular model of SO42- is shown below.Based on the best Lewis electron-dot structure for SO42- and formal charge considerations,what is the predicted S-O bond order for each S-O bond? 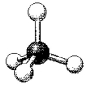
A) 1
B) 1.33
C) 1.5
D) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q147: The carbon-carbon bond in C<sub>2</sub>H<sub>2</sub> contains _
Q148: The hybrid orbital used by nitrogen to
Q149: What is the geometry around the central
Q150: The number of sp<sup>2</sup> hybrid orbitals on
Q151: Shown below is a model of SiH<sub>4</sub>
Q153: What is the geometry around the central
Q154: What is geometry around the nitrogen atom?<br>A)bent<br>B)tetrahedral<br>C)trigonal
Q155: The following ball-and-stick molecular model is a
Q156: Which of the following molecules has polar
Q157: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>Cl<br>B)KCN<br>C)I<sub>2</sub><br>D)H<sub>2</sub>S