Multiple Choice
Which best indicates the direction of the dipole moment in formaldehyde,H2C=O? 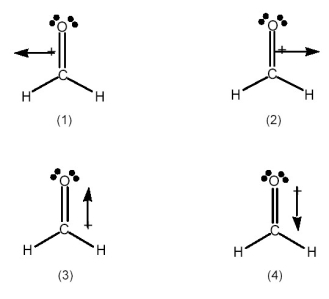
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: The Br-Cl bond has 5.05% ionic character
Q17: The VSEPR model predicts the O-S-O bond
Q18: According to molecular orbital theory,is the highest
Q19: What are the bond angles in the
Q20: What is the geometry around the central
Q22: Which drawing best shows the molecular polarity
Q23: The orbital hybridization on the carbon atom
Q24: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -In the drawing
Q25: What is the hybridization of the carbon
Q26: In which will the O-O bond be