Multiple Choice
Which drawing represents a π* antibonding molecular orbital for a homonuclear diatomic molecule?
A) 
B) 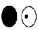
C) 
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q159: The SO<sub>3</sub> molecule can be described as
Q160: The following ball-and-stick molecular model is a
Q161: A molecular model of NO<sub>3</sub><sup>-</sup> is shown
Q162: When an electron is added to the
Q163: What is the molecular geometry of TeF<sub>5</sub><sup>-</sup>?<br>A)octahedral<br>B)seesaw<br>C)square
Q165: The hybrid orbital used by carbon to
Q166: Which molecular orbitals for homonuclear diatomic molecules
Q167: Which of the following should have the
Q168: The orbital hybridization on the central carbon
Q169: Which compound,shown with its dipole moment,is expected