Multiple Choice
Which drawing represents the molecular orbital containing the highest energy electrons in the O22- molecular ion in its the ground state?
A) 
B) 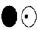
C) 
D) 
Correct Answer:

Verified
Correct Answer:
Verified
Q119: Which molecular orbital resembles a p-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q120: What is the molecular geometry of SCl<sub>4</sub>?<br>A)seesaw<br>B)square
Q121: What are the bond angles in the
Q122: What is the molecular geometry of SbCl<sub>3</sub>?<br>A)T-shaped<br>B)tetrahedral<br>C)trigonal
Q123: The following ball-and-stick molecular model is a
Q125: Which of the following should have the
Q126: Are the π-bonds in CH<sub>3</sub>CO<sub>2</sub><sup>-</sup> delocalized or
Q127: Which molecule has a central atom that
Q128: Which drawing represents the molecular orbital containing
Q129: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>F<sub> </sub><br>B)HCl<sub>