Multiple Choice
What is the geometry around the central atom in the following molecular model of CCl4? 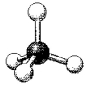
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral
Correct Answer:

Verified
Correct Answer:
Verified
Q5: Molecular orbitals extending over more than two
Q6: What is the molecular geometry of ICl<sub>4</sub><sup>-</sup>?<br>A)seesaw<br>B)square
Q7: Of BrF<sub>3 </sub>and PF<sub>3,</sub>the one with the
Q8: What is the geometry around the central
Q9: Predict whether removing two electrons from F<sub>2</sub>
Q11: AgCl is found to have 78.1% ionic
Q12: Which of the following compounds exhibits only
Q13: Which molecular orbital resembles a d-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q14: Which of the following compounds exhibits hydrogen
Q15: Which covalent bond is the most polar?<br>A)H-I<br>B)H-F<br>C)H-Cl<br>D)H-Br