Multiple Choice
What are the bond angles in the following molecular model of SiCl4? 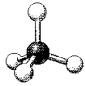
A) less than 109.5°
B) 109.5°
C) less than 120° but greater than 109.5°
D) 120°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q78: Of NH<sub>4</sub><sup>+</sup><sub> </sub>and NH<sub>4</sub><sup>-</sup><sub> </sub>the one with
Q79: Which orbital hybridization is associated with a
Q80: What are the bond angles in the
Q81: Shown below is a model of SF<sub>6</sub>
Q82: Which atomic orbitals are involved in bonding
Q84: What is the hybridization of the carbon
Q85: The HI bond has a length of
Q86: What is the F-B-F bond angle in
Q87: What is the electron geometry and molecular
Q88: Which of the following compounds has the