Multiple Choice
What are the bond angles in the following molecular model of PCl5? 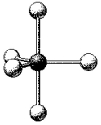
A) some less than 90° and one less than 180°
B) 90° and 180°
C) some less than 90° and some less than 120° but greater than 90°,and one less than 180° but greater than 120°
D) 90°,120°,and 180°
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q93: LiH has an experimental dipole moment,μ =
Q94: Which drawing represents the lowest energy unoccupied
Q95: What are the bond angles in the
Q96: The paramagnetism of O<sub>2</sub> is explained by<br>A)coordinate
Q97: What is the smallest bond angle in
Q99: What are the bond angles in the
Q100: What are the bond angles in the
Q101: The intermolecular forces formed when KI is
Q102: A molecular model of BeF<sub>2</sub> is shown
Q103: What is the geometry around the central