Short Answer
Pt(NH3)2Cl2 is square planar.The isomer of Pt(NH3)2Cl2 that has a non-zero dipole moment has a 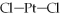 bond angle of ________ degrees.
bond angle of ________ degrees.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: Given that O<sub>2</sub> is paramagnetic and has
Q39: Which drawing represents a σ bonding molecular
Q40: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -In the drawing
Q41: What is geometry around the carbon atom
Q42: Which of the following molecules does not
Q44: What is the bond angle in the
Q45: Which statement concerning any homonuclear diatomic molecule
Q46: Compare the energies of molecular orbitals of
Q47: Which covalent bond is the most polar?<br>A)Cl-F<br>B)B-F<br>C)O-F<br>D)F-F
Q48: The molecular geometry of OCCl<sub>2</sub> is _.