Multiple Choice
Use the given standard enthalpies of formation to calculate ΔH° for the following reaction 3 Fe2O3(s) + CO(g) → 2 Fe3O4(s) + CO2(g) . 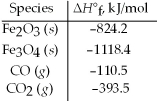
A) -5213.4 kJ
B) -577.2 kJ
C) -47.2 kJ
D) +47.2 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q153: The product,Z,is represented by<br>A)arrow A.<br>B)line D.<br>C)line E.<br>D)line
Q154: For the reaction: A + 2 B
Q155: When 2.00 mol of benzene is vaporized
Q156: At constant pressure for the reaction shown
Q157: The reaction 4 Ag(s)+ O<sub>2</sub>(g)→ 2 Ag<sub>2</sub>O(s)favors
Q159: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What are the
Q160: Find ΔH for BaCO<sub>3</sub> (s)→ BaO (s)+
Q161: How much heat is absorbed when 30.00
Q162: A process is carried out at constant
Q163: He gas is contained in a one-liter