Multiple Choice
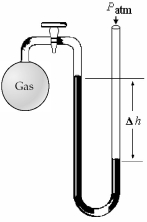
-What is the pressure (in mm Hg) of the gas inside the above apparatus if the outside pressure,Patm,is 736 mm Hg and the difference in mercury levels,Δh,is 18 mm Hg?
A) 18 mm Hg
B) 718 mm Hg
C) 736 mm Hg
D) 754 mm Hg
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q74: Each of three identical 15.0-L gas cylinders
Q75: One mole of which gas has the
Q76: A 1.75-L container filled with CO<sub>2</sub> gas
Q77: What is the density of chlorine gas
Q78: The partial pressures of CH<sub>4</sub>,N<sub>2</sub>,and O<sub>2</sub> in
Q80: If mercury (density = 13.6 g/cm<sup>3</sup>)at a
Q81: A lungful of air (500 mL)contains 4.1%
Q82: How many grams of XeF<sub>6</sub> are required
Q83: A 0.500 g sample containing Ag<sub>2</sub>O and
Q84: Which of the following gases has the