Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the number of moles of gas while keeping the pressure and temperature constant? 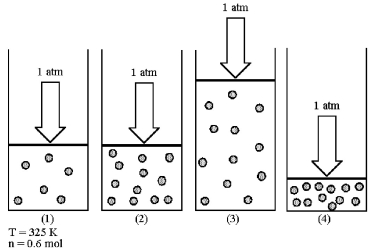
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q178: What is the pressure in a gas
Q179: A 55.0-L steel tank at 20.0°C contains
Q180: Which of the following regions of the
Q181: Given three cylinders containing O<sub>2</sub> gas at
Q182: A 1.000 kg sample of nitroglycerine,C<sub>3</sub>H<sub>5</sub>N<sub>3</sub>O<sub>9</sub>,explodes and
Q183: Each of three identical 15.0-L gas cylinders
Q184: At STP how many liters of NH<sub>3</sub>
Q185: A 0.429-g sample of gas occupies 125
Q186: Assume that you have a mixture of
Q188: In the laboratory,hydrogen gas is usually made