Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the temperature while keeping the pressure and number of moles of gas constant? 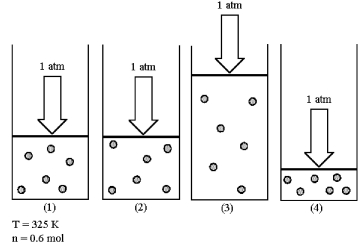
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q38: A basketball is inflated to a pressure
Q39: According to the kinetic molecular theory of
Q40: A gas occupies 22.4 L at STP
Q41: Two moles of neon gas at 20.0°C
Q42: The action of some commercial drain cleaners
Q44: According to the kinetic molecular theory of
Q45: Three bulbs,two of which contain different gases
Q46: A gas occupies 22.4 L at STP
Q47: Cyanogen is a gas which contains 46.2%
Q48: At what temperature will sulfur hexafluoride molecules