Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram. 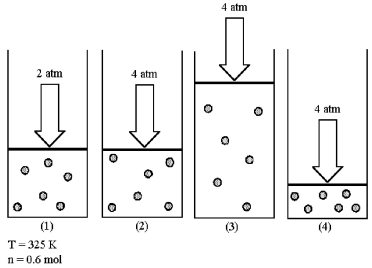
-Which diagram (2) -(4) most closely represents the result of doubling the pressure while keeping the temperature and number of moles of gas constant?
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer:

Verified
Correct Answer:
Verified
Q152: At STP the number of liters of
Q153: Under the same pressure and temperature conditions,the
Q154: A 5.00-L flask contains nitrogen gas at
Q155: In the diagram below,helium atoms are represented
Q156: Historical records of greenhouse gases can be
Q158: What is the volume of 20.0 g
Q159: Which one of the following gases will
Q160: In the diagram below,helium atoms are represented
Q161: What is the total pressure in a
Q162: When 30.0 g of zinc metal reacts