Multiple Choice
Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 20 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 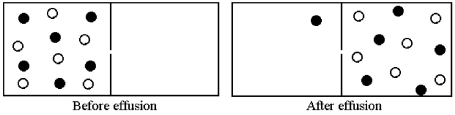
A) Unshaded molecules have higher average speed and molecular mass = 14 amu.
B) Unshaded molecules have higher average speed and molecular mass = 17 amu.
C) Unshaded molecules have lower average speed and molecular mass = 24 amu.
D) Unshaded molecules have lower average speed and molecular mass = 29 amu.
Correct Answer:

Verified
Correct Answer:
Verified
Q87: For an ideal gas,which pairs of variables
Q88: Each of three identical 15.0-L gas cylinders
Q89: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q90: A steel bottle contains argon gas at
Q91: How many grams of XeF<sub>6</sub> are required
Q93: In the diagram below,nitrogen molecules are represented
Q94: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q95: Which of the following would have a
Q96: A 1.75 L container filled with CO<sub>2</sub>
Q97: An "empty" aerosol can at 25°C still