Multiple Choice
The picture shown below represents a two-dimensional lattice of atoms M and X. 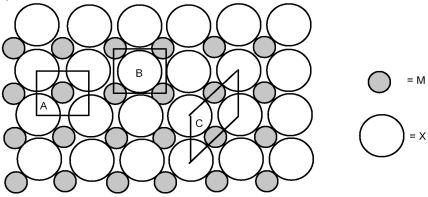
-What is the formula of the compound formed from M and X?
A) MX
B) MX2
C) MX3
D) MX4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: A binary ionic compound,M<sub>x</sub>A<sub>y</sub>,crystallizes in a cubic
Q53: Barium has a radius of 224 pm
Q54: Ni has a face-centered unit cell.The number
Q55: Which of the intermolecular forces is the
Q56: What phase changes occur when the pressure
Q58: How much heat is released when 125.0
Q59: Solids having no ordered long-range structure are
Q60: The normal boiling point for HBr is
Q61: How many atoms are in one body-centered
Q62: Use the diagram below to answer the