Multiple Choice
A certain mineral crystallizes in the cubic unit cell shown below. 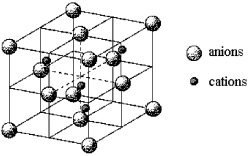
-What kind of packing do the anions adopt?
A) body-centered cubic
B) cubic closest packed (face-centered cubic)
C) hexagonal closest packed
D) simple cubic
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q100: What phases can be present at 200°C
Q101: The normal boiling point of this substance
Q102: What is the physical phase of the
Q103: Which is expected to have the highest
Q104: A certain mineral,M<sub>x</sub>M'<sub>y</sub>A<sub>z</sub>,crystallizes in the cubic unit
Q106: Manganese crystallizes in a body-centered cubic structure.What
Q107: A kitchen pressure cooker operates at 1.70
Q108: Which transition could occur if a solid
Q109: Lead has a radius of 154 pm
Q110: Which of the following forms a metallic