Multiple Choice
Use the diagram below to answer the following questions. 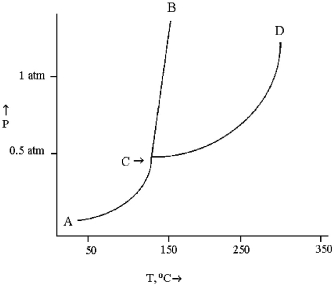
-The solid and liquid phases can exist in equilibrium along line
A) AC.
B) CB.
C) CD.
D) BD.
Correct Answer:

Verified
Correct Answer:
Verified
Q16: The phase change H<sub>2</sub>(g)→ H<sub>2</sub>(s)is called _,and
Q17: First-order diffraction of X-rays with d =
Q18: Which of the following forms an ionic
Q19: Of C<sub>2</sub>H<sub>5</sub>OH and C<sub>3</sub>H<sub>5</sub>(OH)<sub>3</sub> the one expected
Q20: Ethyl chloride,C<sub>2</sub>H<sub>5</sub>Cl,is used as a local anesthetic.It
Q22: The highest coordination number for spherical packing
Q23: When a narrow diameter glass tube is
Q24: Identify the packing in the figure shown
Q25: Hydroquinone is an antioxidant that is also
Q26: What phase changes occur when the temperature