Multiple Choice
Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 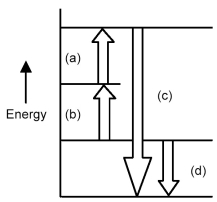
-Which arrow represents ΔHsoln?
A) arrow (a)
B) arrow (b)
C) arrow (c)
D) arrow (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q35: A solution is prepared by dissolving 17.75
Q36: Which cation in each set is expected
Q37: What is the mole fraction of I<sub>2</sub>
Q38: When solute is added to water,the solution
Q39: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which drawing above
Q41: A solution of LiCl in water has
Q42: KI does not dissolve well in nonpolar
Q43: A solution of a nonelectrolyte solution contains
Q44: Most gases become less soluble in water
Q45: To make a 3.00 m solution,one could