Multiple Choice
Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 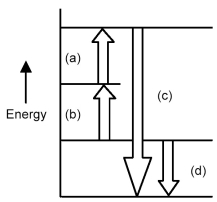
-Which arrow represents ΔHsolute-solvent?
A) arrow (a)
B) arrow (b)
C) arrow (c)
D) arrow (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q52: The coolant in automobiles is often a
Q53: A solution has a density of 1.023
Q54: How many grams of sucrose,C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>,must be added
Q55: At a given temperature the vapor pressures
Q56: Molarity is defined as moles of solute
Q58: At a given temperature the vapor pressures
Q59: To make a 3.0 M solution,one could
Q60: A 0.50 m solution of which solute
Q61: At 20°C and 0.28 atm pressure Xenon
Q62: A solution is prepared by dissolving 17.75