Multiple Choice
Arrows in the energy diagram below represent enthalpy changes occurring in the exothermic formation of a solution:
ΔHsoln = enthalpy of solution
ΔHsolute-solute = enthalpy change involving solute-solute interactions
ΔHsolute-solvent = enthalpy change involving solute-solvent interactions
ΔHsolvent-solvent = enthalpy change involving solvent-solvent interactions 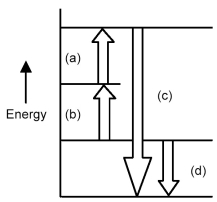
-Which arrows represent ΔHsolute-solute and ΔHsolvent-solvent?
A) arrow (a) and arrow (b)
B) arrow (a) and arrow (c)
C) arrow (a) and arrow (d)
D) arrow (c) and arrow (d)
Correct Answer:

Verified
Correct Answer:
Verified
Q172: Calculate the freezing point of a solution
Q173: When an ionic solute dissolves in water
Q174: When two similar liquids mix to form
Q175: A 0.020 m aqueous solution containing which
Q176: A 3.17 m solution of CaCl<sub>2</sub> in
Q178: Which of the following solutions will have
Q179: A solution is made by dissolving 19.5
Q180: How many grams of KBr are required
Q181: Red blood cells are placed into pure
Q182: The average osmotic pressure of blood is