Multiple Choice
Drawing (1) shows a nonequilibrium system comprised of pure water separated from an aqueous solution by a semipermeable membrane.Shaded spheres represent solute particles and unshaded spheres represent water molecules.Which drawing (2) -(5) represents this system after equilibrium is reached? 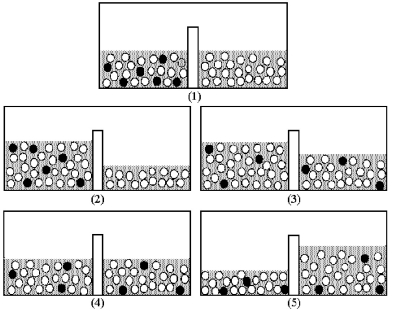
A) drawing (2)
B) drawing (3)
C) drawing (4)
D) drawing (5)
Correct Answer:

Verified
Correct Answer:
Verified
Q137: What molality of pentane is obtained by
Q138: At 80.0°C heptane,C<sub>7</sub>H<sub>16</sub>,has a vapor pressure of
Q139: A KCl solution is prepared by dissolving
Q140: Iodine,I<sub>2</sub>(s),is more soluble in dichloromethane,CH<sub>2</sub>Cl<sub>2</sub>(l),than in water
Q141: Which is not a solution?<br>A)sterling silver<br>B)fog<br>C)hydrochloric acid<br>D)coffee
Q143: A 1.40 M solution of CaCl<sub>2</sub> in
Q144: A gold ring is an example of
Q145: For which case would ΔH<sub>soln</sub> be expected
Q146: The volume of 0.200 M H<sub>2</sub>SO<sub>4</sub> that
Q147: When KI is dissolved in water,the major