Multiple Choice
A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 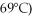 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 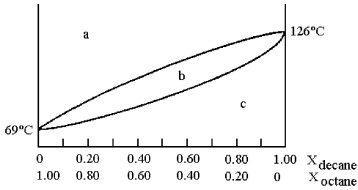
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what region of the diagram corresponds to liquid?
A) region a
B) region b
C) region c
D) regions a and c
Correct Answer:

Verified
Correct Answer:
Verified
Q85: Which of the following does not affect
Q86: A solution of LiCl in water is
Q87: To make a 0.125 m solution,one could
Q88: A 1.0 m aqueous BaI<sub>2</sub> solution will
Q89: Drawing (1)shows the equilibrium vapor pressure of
Q91: What volume of 3.00 M CH<sub>3</sub>OH solution
Q92: A solution of LiCl in water is
Q93: The following diagram shows a close-up view
Q94: What molality of pentane is obtained by
Q95: Which cation in each set would be