Multiple Choice
A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 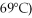 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 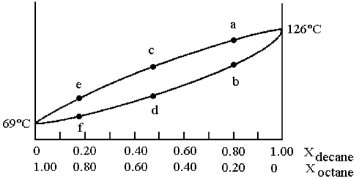
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,at what approximate temperature will the mixture begin to boil?
A) temperature at point a
B) temperature at point b
C) temperature at point d
D) temperature at point f
Correct Answer:

Verified
Correct Answer:
Verified
Q164: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -What is the
Q165: If dissociation of MgCl<sub>2</sub> in water were
Q166: The vapor pressure of water at 25°C
Q167: In the process of dissolving ionic compounds,the
Q168: In which case should CO<sub>2</sub>(g)be more soluble
Q170: Molarity is defined as _,whereas molality is
Q171: What volume of 3.00 M CH<sub>3</sub>OH solution
Q172: Calculate the freezing point of a solution
Q173: When an ionic solute dissolves in water
Q174: When two similar liquids mix to form