Multiple Choice
A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 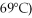 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 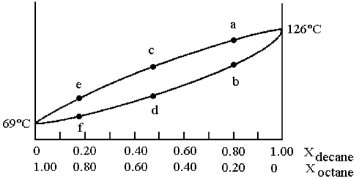
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what is the liquid composition at the boiling point?
A) 100% decane
B) composition at point b
C) composition at point c
D) composition at point e
Correct Answer:

Verified
Correct Answer:
Verified
Q104: A solution is prepared by dissolving 20.0
Q105: The Henry's Law constant of methyl bromide,CH<sub>3</sub>Br,is
Q106: What are the major solute-solvent interactions created
Q107: Naproxen is a commercially important anti-inflammatory agent
Q108: What is the mole fraction of ethanol
Q110: In which case should CO<sub>2</sub>(g)be more soluble
Q111: To make a 0.125 M solution,one could
Q112: At 25.0°C,a solution has a concentration of
Q113: A phase diagram of temperature versus composition
Q114: The solubility of argon in water at