Multiple Choice
A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 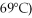 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 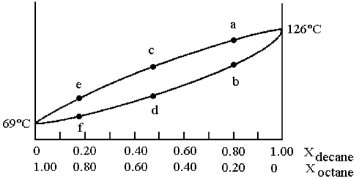
-Assume that the vapor at point c is condensed and reboiled.What is the liquid composition of the condensed vapor prior to reboiling?
A) 100% decane
B) composition at point b
C) composition at point d
D) composition at point e
Correct Answer:

Verified
Correct Answer:
Verified
Q3: A phase diagram of temperature versus composition
Q4: Which concentration becomes smaller as the temperature
Q5: Fresh air contains approximately 400 ppm CO<sub>2</sub>,whereas
Q6: What is the mole fraction of oxygen
Q7: Molality is defined as moles of solute
Q9: The normal boiling point of pure benzene
Q10: The rubbing alcohol sold in drug stores
Q11: What is the mole fraction of ethanol
Q12: When 2.36 g of a nonvolatile solute
Q13: The osmotic pressure of a 0.10 M