Multiple Choice
At 80°C,pure liquid A has a vapor pressure of 700 mm Hg and pure liquid B has a vapor pressure of 940 mm Hg.What is XA for a solution of A and B with a normal boiling point of 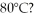
A) 0.25
B) 0.50
C) 0.75
D) A solution of A and B cannot boil at 80°C.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q119: Which should be least soluble in water?<br>A)<br><img
Q120: Which of the following solutions will have
Q121: A 0.35 m aqueous solution of an
Q122: What are the major solute-solvent interactions created
Q123: The change in the Gibbs free energy
Q125: An aqueous CsCl solution is 8.00 wt%
Q126: What volume of 0.716 M KBr solution
Q127: A solution of LiCl in water has
Q128: A solution is prepared by dissolving 17.75
Q129: A 2.00 M solution of CaCl<sub>2</sub> in