Multiple Choice
Substances with high lattice energies tend to be less soluble than substances with low lattice energies.On that basis predict the relative aqueous solubility at 20°C,from highest to lowest,of the following ionic compounds: 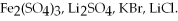
A) Fe2(SO4) 3 > Li2SO4 > KBr > LiCl
B) Fe2(SO4) 3 > Li2SO4 > LiCl > KBr
C) KBr > LiCl > Li2SO4 > Fe2(SO4) 3
D) LiCl > KBr > Li2SO4 > Fe2(SO4) 3
Correct Answer:

Verified
Correct Answer:
Verified
Q145: For which case would ΔH<sub>soln</sub> be expected
Q146: The volume of 0.200 M H<sub>2</sub>SO<sub>4</sub> that
Q147: When KI is dissolved in water,the major
Q148: For a liquid solution made by dissolving
Q149: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which drawing above
Q151: At 25°C the vapor pressures of benzene
Q152: The osmotic pressure of a 100-mL solution
Q153: What is the molality of a glucose
Q154: Which of the following is not an
Q155: Calculate the molality of a solution with