Multiple Choice
Consider the interconversion of A molecules (shaded spheres) and B molecules (unshaded spheres) according to the reaction A ⇌ B.Each of the following series of pictures represents a separate experiment in which time increases from left to right. 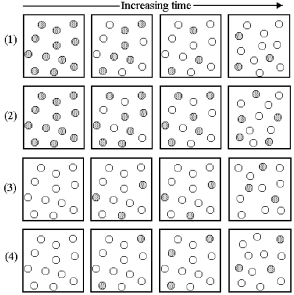
-What is the value of the equilibrium constant Kc for the reaction A ⇌ B?
A) Kc = 0.33
B) Kc = 3.0
C) Kc = 12
D) Kc = 27
Correct Answer:

Verified
Correct Answer:
Verified
Q83: The following two isomers of C<sub>3</sub>H<sub>7</sub>NO exist
Q84: The reaction below virtually goes to completion
Q85: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q86: The hexaammine cobalt(III)ion is very unstable in
Q87: If K<sub>c</sub> = 7.04 × 10<sup>-2</sup> for
Q89: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)=
Q90: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2
Q91: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)⇌
Q92: The pink and blue species below form
Q93: At 298 K,K<sub>p</sub> = 2.1 × 10<sup>4</sup>