Multiple Choice
The following pictures represent mixtures of cis-C2H2X2 molecules and trans-C2H2X2 molecules,which interconvert according to the equation cis-C2H2X2 ⇌ trans-C2H2X2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 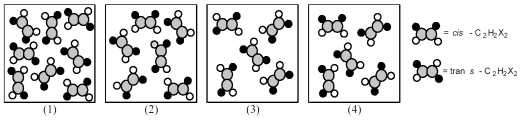
A) mixture (2)
B) mixture (3)
C) mixture (4)
D) None of the other mixtures are at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q46: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q48: At a certain temperature,K<sub>c</sub> equals 1.4 ×
Q49: The reaction below is heated from 20°C
Q50: Nitric oxide reacts with oxygen to form
Q52: Calcium carbonate is relatively insoluble and the
Q53: The equilibrium equation is also known as
Q54: The following picture represents the equilibrium state
Q55: For the isomerization reaction: butane ⇌ isobutane<br>K<sub>p</sub>
Q56: The equilibrium constant K<sub>c</sub><sup> </sup>for the reaction
Q90: For the reaction: N<sub>2</sub>(g)+ 2 O<sub>2</sub>(g)⇌ 2