Multiple Choice
The following pictures represent mixtures of A2B4 molecules and AB2 molecules,which interconvert according to the equation A2B4 ⇌ 2 AB2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 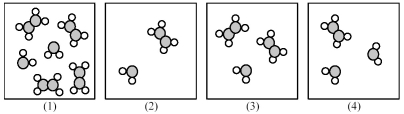
A) mixture (2)
B) mixture (3)
C) mixture (4)
D) None of the other mixtures are at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q123: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q124: Nickel metal can be prepared by the
Q125: The overall reaction for photosynthesis can be
Q126: Shown below is a concentration vs.time plot
Q127: The equilibrium constant is equal to 5.00
Q129: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q130: At a certain temperature the equilibrium constant,K<sub>c</sub>,equals
Q131: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298
Q132: At 1000 K,K<sub>p</sub> = 19.9 for the
Q133: What is the equilibrium equation for the