Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ B.For this reaction the value of the equilibrium constant is 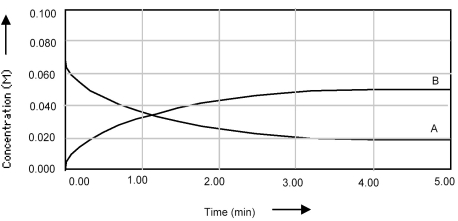
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Q67: Picture (1)represents the equilibrium mixture for the
Q68: Gaseous hydrogen bromide decomposes at elevated temperatures
Q69: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)⇌
Q70: What is true about the relationship of
Q71: For the reaction A<sub>2</sub> + 2 B<sub>3</sub>
Q73: The oxidation of sulfur dioxide by oxygen
Q74: For the reaction shown below,N<sub>2</sub>O<sub>4</sub> and NO<sub>2</sub>
Q75: Salt solubilities can be compared by the
Q76: At some temperature,a 4.0 L flask is
Q77: The equilibrium constant,K<sub>p</sub>,equals 3.40 for the isomerization