Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ 2B.For this reaction the value of the equilibrium constant is 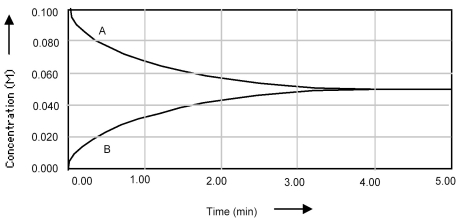
A) Kc < 1.
B) Kc = 0.
C) Kc = 1.
D) Kc > 1.
Correct Answer:

Verified
Correct Answer:
Verified
Q59: Consider the reaction HCO<sub>3</sub><sup>-</sup> (aq)+ H<sub>2</sub>O (l)⇌
Q60: For acid solutions of the same molarity
Q61: The following pictures represent the initial state
Q62: At a certain temperature,bromine and nitric oxide
Q63: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q65: A catalyst increases the rate of a
Q66: If K<sub>c</sub> is the equilibrium constant for
Q67: Picture (1)represents the equilibrium mixture for the
Q68: Gaseous hydrogen bromide decomposes at elevated temperatures
Q69: The decomposition of ammonia is: 2 NH<sub>3</sub>(g)⇌