Multiple Choice
The following pictures represent the initial state and the equilibrium state for the gaseous state reaction of A2 molecules (shaded spheres) with B atoms (unshaded spheres) to give AB molecules. 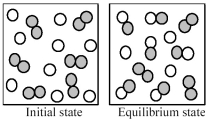
-What is the best balanced chemical equation for the reaction?
A) A2 + B ⇌ A2B
B) A2 + 2 B ⇌ A2B2
C) A2 + 2 B ⇌ 2 AB
D) 6 A2 + 9 B ⇌ 3 A2 + 3B + 6 AB
Correct Answer:

Verified
Correct Answer:
Verified
Q99: K<sub>p</sub> is related to K<sub>c</sub> by the
Q100: For which one of the following reactions
Q101: Which equilibrium below is homogeneous?<br>A)CaSO<sub>4</sub>(s)⇌ Ca<sup>2+</sup>(aq)+ SO<sub>4</sub><sup>2-</sup>(aq)<br>B)2
Q102: The equilibrium constant,K<sub>p</sub>,equals 3.40 for the isomerization
Q103: K<sub>c</sub> = 57.0<sup> </sup>at 700 K for
Q105: Which of the following changes in reaction
Q106: A mixture of carbon monoxide,hydrogen,and methanol is
Q107: If K<sub>c</sub> = 2.0 × 10<sup>33</sup> at
Q108: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q109: K<sub>c</sub> = 1.2 × 10<sup>-42 </sup>at 500